
Abstract
Background. Fms-like tyrosine kinase-3 (FLT3) mutation is found in about 25% to 30% of acute myeloid leukemia (AML) patients, and its associated with a poor prognosis. There are two categories: internal tandem duplication FLT3-ITD, approximately occurred in 25% of patients, and mutations in the tyrosine kinase domain (FLT3-TKD), occurred in 5%-7% of patients.
Objectives. The aim of this work is to evaluate the mutation status of FLT3-ITD at diagnosis and its impact on therapeutic response, relapse and survival.
Patients and methods. FLT3-ITD mutation was carried out by qualitative Polymerase Chain Reaction (PCR) at diagnosis of AML. Detailed clinical, biological and therapeutic patient information was collected from the recorded database. Subsequently AML patients were divided into 2 groups (G); G1: mutated FLT3-ITD AML and G2: wild-type FLT3-ITD AML. All patients received induction type 3+7 (daunorubicin + cytarabine), with in case of complete remission (CR), followed by cycles of consolidation, or
allogeneic hematopoietic cell transplantation (allo-HCT). Sorafenib was combined to induction chemotherapy, consolidation and maintenance post allo-HCT depending on product availability in AML FLT3-ITD patients.
Results. From 2014 to 2021, 127 AML patients were tested for FLT3-ITD. 25 pts (20%) presented with an FLT3-ITD type mutation (G1), and 102 pts (80%) with wild-type FLT3-ITD (G2). The presence of FLT3-ITD mutation did not affect the achievement of remission 76% verus 74 % (p=0.8), relapse rate 47% versus 45% (p=0.77). Relapse-free survival and overall survival were 36 months and 15 months shorter in the G1 (mutated FLT3-ITD) than in G2 (wild-type FLT3-ITD). However, these differences were not statistically difference between two groups p=0.10 and p= 0.14 respectively.
Conclusion. Mutated FLT3-ITD have a poor prognosis in AML patients, require an appropriate therapeutic strategy. New anti FLT3 inhibitors as midostaurin, and gilteritinib are needed to optimize the care of these patients.
Introduction
Acute myeloid leukemia (AML) is a complex, dynamic malignancy characterized by multiple acquired somatic mutations and coexisting competing clones (1). The leukemogenesis hypothesis implies that AML is the consequence of at least two mutations, one conferring a proliferative advantage (class I mutations), and another impairing hematopoietic differentiation (class II mutations) (2, 3). Type I mutations include for example; Fms-like tyrosine kinase 3 (FLT3), Kirsten rat sarcoma (K-RAS) and tyrosine-protein kinase Kit (KIT) mutations, while mutations in CCAAT/enhancer-binding protein alpha (CEBPA) are type
abnormalities II. FLT3 is a tyrosine kinase receptor that plays a key role in cell survival, proliferation and differentiation of hematopoietic stem cells. The FLT3 mutation is found in about 25 to 30% of AML, associated with a normal karyotype (about 40%) (4). It confers a poor prognosis with a high risk of relapse (5, 6). There are two categories: internal tandem duplication (ITD) in the juxta membrane domain of the receptor (FLT3-ITD, about 25%). Point mutations resulting in single amino acid substitutions in the tyrosine kinase domain (TKD) activation loop (FLT3-TKD), approximately 5% to 7%(4). The aim of this work is to evaluate the mutation status of FLT3-ITD at diagnosis and its impact on therapeutic response, relapse and survival in patients with AML.
Patients and methods
Patients
This is a monocentric retrospective study conducted at the hematology and cell therapy department, and in collaboration with the cytogenetics and molecular biology department at the EHU 1er Novembre in). Oran. From 2014 we introduced the search for FLT3-ITD mutation status in all patients with newly diagnosed AML. Detailed clinical, biological and therapeutic patient information was collected from the service’s database. Subsequently the patients with AML were divided into 2 groups; group 1 (G1): (mutated FLT3-ITD) and G2 (wild-type FLT3-ITD
FLT3 mutation research methods
The search for the FLT3-ITD mutation was carried out by qualitative Polymerase Chain Reaction (PCR), followed by agarose gel electrophoresis. DNA was extracted from peripheral blood or bone marrow with the Maxwell 16 automatic system (Promega Corporation, Madison, WI). FLT3-ITD mutation detection was performed by amplifying exons 14 and 15 with primers specific for the FLT3 gene region using the Seeplex FLT3 Genotyping Kit (Seegene, Rockville, MARYLAND). Subsequently, an electrophoretic analysis of the amplification products was carried out in an agar gel.
Treatment
All patients received induction type 3+7 (daunorubicin 90mg/m2 for 3 days, cytarabine 100mg/m2 for 7 days) with in case of complete remission (CR), followed by cycles of consolidation with high (3g/m3/12H days 1, 3, 5) or intermediate dose of cytarabine (1.5g/m3/12h days 1, 2, 3), and
an allogeneic hematopoietic cells transplantation (allo-HCT). Sorafenib was combined from day eight of induction chemotherapy and day six of consolidation depending on product availability in AML patients with mutated FLT3-ITD. Patients with acute promyelocyte leukemia (AML3) were treated according to the APL 2000 protocol.
Evaluation criteria and statistical analyzes
The primary objective was to assess the frequency of FLT3-ITD mutation status at the diagnosis of AML patients. The secondary objectives were focused on the comparison between the two groups; G1: (mutated FLT3-ITD) and G2 (wild-type FLT3-ITD) in terms of response, relapse rate, relapse-free survival and overall survival.
The comparison between the two groups was carried out by the Student test. Survival curves were calculated using the Kaplan–Meier method. The study point date is May 30, 2022.
Results
Patient characteristics and frequency of FLT3-ITD mutation status:
From January 2014 to December 31, 2021, 127 patients with AML were included. Among the 127 patients having been the subject of an FLT3-ITD search, 102 pts (80%) presented a wild-type FLT3-ITD gene (G1) with a median age of 36 (16-64) including 51 mal and 49 females, ratio=1.04. The average levels of Wight blanc count (WBC), hemoglobin (Hb), platelets are respectively 7.1 G/L, 8 g/dl, 46 G/L. 25 (20%) patients presented a mutated FLT3-ITD AML (G2) (Figure. 1). The median age was 36 years (15-63), male=16, female=9, ratio=1.77. The average levels of WBC, Hb, platelets are respectively 23 G/L (p=0.7), 8.6 g/dl (p=0.9), 32 G/L (p=0.13). The FLT3-ITD mutation was found in all subgroups of the French-American-British classification (Figure 2), it is more frequent in AML 3 and AML 2.
In G1, the karyotype was available in 17pts, the FLT3 mutation was associated with a normal karyotype in 47%, and a t(15-17) in 29%. In G2, the karyotype was available in 56 patients (38% normal karyotype, 18% favorable, 27% intermediate 2, and 16% unfavorable) (Figure 3). Table 1 summarizes the clinical and biological characteristics of the two groups.
Response to treatment, relapse rate:
In terms of response after the first induction, we note 65/85 (76%) achieved CR in G1 and 17/23 (74%) in
G2 (p=0.80) (figure 4). The relapse rate was 29/65 (45%) in G1 and 8/17 (47%) in G2 (p=0.77) (Figure 5). The CR and relapse rate in patiens (N=8) who received sorafenib and those who did not (N=16) were 88% and 37%, versus 69% (p=0.33) and 55% (p=0.65) respectively (Figure 6).
Relapse-free survival and overall survival:
Median relapse-free survival (RFS) and OS were 49 months versus 13 months (p=0.1), and 29 months versus 14 months in G2 (mutated FLT3-ITD) and G1 (wild-type FLT3-ITD) respectively (p= 0.149). RFS and OS were 36 months and 15 months shorter in the G2 than in G1 (Figure 7, Figure 8).
Discussion
In Algeria, hematological malignancies in adults represent 10% of all cancerous pathologies. Four national surveys carried out over four different periods show that the incidence of AML is continuously increasing, with an estimated incidence between 2018-2021 of 1.29/100,000 inhabitants and a prevalence of 1584 cases (400 new cases/year) (7- 10), with a median age at diagnosis of 49 years. This is the first nationwide study to assess the frequency and impact of FLT3-ITD mutation status on treatment response and survival in patients with AML. In our series, the incidence of the FLT3-ITD mutation was 20% similar to that reported in other countries; 22% in the USA, 17% in India, 23% in Japan, and 23% in Germany (11-14). In addition, 28% of patients with mutated FLT3-ITD AML had AML as French–American–British classification (FAB), and 20% AML2 type, which had the highest incidence of mutations of all FAB subtypes. The FLT3 mutation is associated with a high tumor burden, higher leukocyte count (23 g/L) versus 7.1 g/L for patients without FLT-ITD mutation, however this difference was not statistically different (p=0.9). In a recent Indian study, Shankaralingappa et al, reported a higher tumor burden in FLT3 mutation AML with higher leukocyte count, percentage of peripheral and medullary blasts, higher LDH count. These data have been confirmed by other studies (15, 16). The high tumor burden in FLT3-ITD-positive AML patients can be attributed to a ligand-independent constituent element activation of the FLT3 kinase. This activates signaling of downstream
molecules that provide a survival advantage and drive cell myeloproliferation (17, 18). We analyzed in detail the incidence of mutated FLT3-ITD AML patients in cytogenetic AML subgroups, and as already described in the literature, the FLT3 mutation is more frequent in pts with a normal karyotype (12). In our study, the presence of an FLT3-ITD mutation did not predict the achievement of CR, which is in agreement with the data from the MRC AML 12 and 13 study (19), and another German study (14). In our series, the presence of the FLT3-ITD mutation had an impact on the long-term results of the patients, with a median of relapse-free survival, and shorter overall survival 13 months against 49 months, and 14 months against 29 months in the wild-type FLT3-ITD AML group, but this difference was not statistically different, probably wild-type FLT3-ITD AML group, but this difference was not statistically different, probably related to the small number of patients recruited. The lack of effect on the CR rate may be due to the fact that the FLT3-ITD mutation does not significantly affect chemosensitivity, but also to the use in 5 patients of sorafenib, a 1st generation type II multi-kinase inhibitor anti-FLT3.
Given its availability, numerous clinical trials have evaluated the addition of sorafenib to the standard chemotherapy in the first-line induction treatment of AML patients, with controversial results (20-23) Rollig et al reported a randomized placebo-controlled trial (SORAML) of adding sorafenib to standard induction-consolidation therapy in 267 patients with newly diagnosed AML aged 60 or younger, independently to the FLT3 mutation status. The addition of sorafenib led to an improvement the 3 years EFS, 40% with sorafenib versus 22% in the placebo group (p=0.013). However, no improvement in overall survival was observed, probably related to the number of allograft patients in the placebo arm (21). The most frequently reported adverse events during treatment with sorafenib were fatigue, grade 1 diarrhea, palmar-plantar erythrodysaesthesia, and elevated transaminases. Other anti FLT3 ITD inhibitors with broader kinase activity (anti FLT3-ITD and TKD) are currently approved by the European medicine agency and the Food drug approved such as midostaurin in newly diagnosed mutated FLT3 AML patients in combination with induction and consolidation chemotherapy (24), or gilteritinib in
relapsed or refractory patients (25, 26).
Conclusion
Patients with mutated FLT3-ITD AML have a poor prognosis, and require an adapted therapeutic strategy, which underlines the importance of acquiring new anti-FLT3 inhibitors in the future such as: midostaurin, and gilteritinib.
References
Perl AE, Larson RA. Follow-up of patients with R/R FLT3-mutation-positive AML treated with gilteritinib in the phase 3 ADMIRAL trial. 2022;139(23):3366-75.
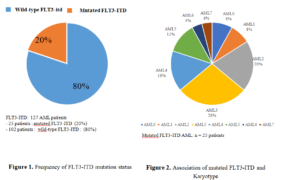
Table 1. Patients characteristic
|
Patients characteristics |
G1
Wild-type FLT3-ITD |
G2
Mutated FLT3-ITD |
P value |
| Patients, number | 102 (80%) | 25 (20%) |
– |
| Age, median, range | 36 (16-64) | 36 (15- 63) |
– |
| Gender: Male/Female, ratio | 51/49, (1,04) | 16/9, (1,77) |
0,21 |
|
White blood count (G/L), median |
7,1 |
23 |
0.9 |
|
Hemoglobin (g/dl), median |
8 |
8,6 |
0.9 |
|
Platelet (G/L), median |
46 |
32 |
0.13 |
|
Cytogenetic – Favorable – Intermediate – Adverse |
n=56
10 (18%) 37 (65%) 9 (16%) |
n=17
5 (29%) 8(47%) 3 (18%) |
0,22 0,20 0,93 |
|
Anti FLT3 treatment AML (induction) AML (selvage ) |
– – |
8 (32%) 5 3 |
|
| Allogeneic HCT, number |
28 (27%) |
5 (20%) |
0,45 |

